UMMC seeks participants for immunity supplement study
A new natural product clinical trial is underway at the University of Mississippi Medical Center, and researchers are urgently recruiting participants.
Dr. Gailen Marshall, the R. Faser Triplett Sr. MD Chair of Allergy and Immunology, is principal investigator of the UMMC arm of the double-blind study he designed in collaboration with the National Center for Natural Products Research, part of the School of Pharmacy at the University of Mississippi.
The study is funded by a $5.6 million grant from the National Institutes of Health.
Immulina, patented by Ole Miss and already available for purchase, is an extract of the spirulina, a blue-green alga that became popular after NASA astronauts used it as a dietary supplement on space missions. Based on several studies, it can boost the immune system and reduce inflammation by preventing the release of a histamine.
When the grant was awarded in 2020, researchers were interested in its effects on influenza.
They spent the first two years “advancing the chemistry research, investigating potential biomarkers and mouse studies on antiviral resilience. This research was important in helping to provide the foundation for conducting the current human study,” said Nirmal Pugh, NCNPR principal scientist.

“But the results of its work could impact how we might be able to protect ourselves against respiratory viruses in the future whether it is flu, CoV-2 or other future viruses that could cause a new pandemic,” said Marshall.
To volunteer, you must be at least 18 with no recent changes in your health and no active autoimmune disease. As long as someone is generally healthy, an illness will not necessarily preclude participation as long as it is controlled, said Marshall.
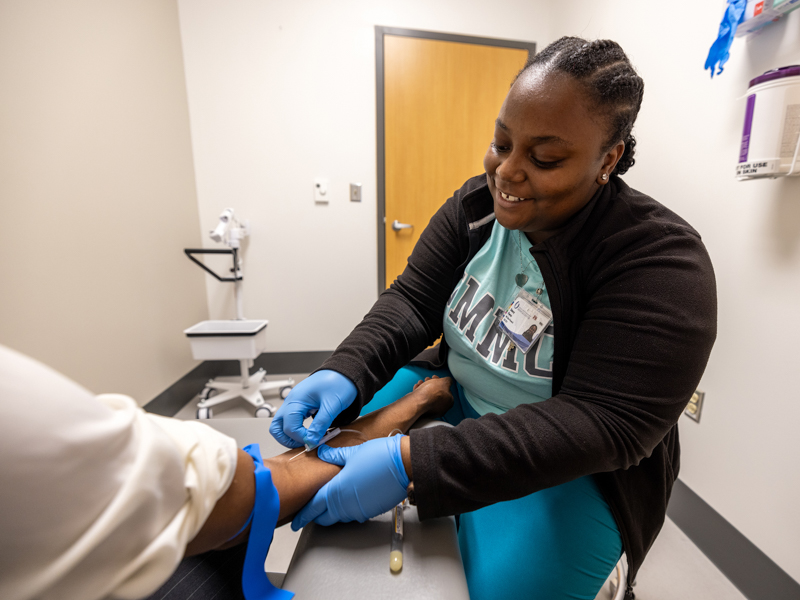
Here’s what volunteers can expect: They will undergo an initial screening that consists of a questionnaire, and if deemed eligible, receive either Immulina or placebo capsules to take twice a day. Participants must consent to 12 visits over a 20-week period. At each visit, researchers will draw 3 tablespoons of blood to measure specific cells of the immune system.
Volunteers given the Immulina tablets will receive one of three dosages: 200, 400 or 800 milligrams, said Joy Walker, research specialist.
After each visit, volunteers will receive $25, for a total of $300. All visits will take place in the Clinical Research and Trials Unit on 7 South of the adult hospital.
Marshall wants to enroll 472 volunteers, who can begin the process by emailing ImmulinaFlu@umc.edu.
The trial is “somewhat unique in itself because we are not looking for a cure directly or antiviral activity of the Immulina, but rather increasing resiliency in the human body to fight the infection,” said Dr. Ikhlas Khan, NCNPR director and distinguished research professor of pharmacognosy at Ole Miss.
“The outcome of this study will provide well-needed evidence that natural products have beneficiary effects on human health. We are excited since it will be conducted using Immulina, which our scientists developed.”


