IDEAS, clinical trials come to MIND Center
Count backwards from 100 by sevens. Ninety-three, eighty-six, seventy…eight? No, seventy- nine.
When Brenda Hankins of Pelahatchie visited her general practitioner this year, she was asked to do just that after sharing some concerns.
“I'm not a big complainer, but I told my doctor how I used to be the first person up and going in the morning and it was harder to do that now,” said Hankins, 67. “But the thing that made me mad was that I couldn't remember things like I used to,” she said.
Hankins has always been on the go. She has visited all 48 contiguous states, hiked the entire Appalachian Trail and recently returned from a three-week trip to the Great Smoky Mountains.
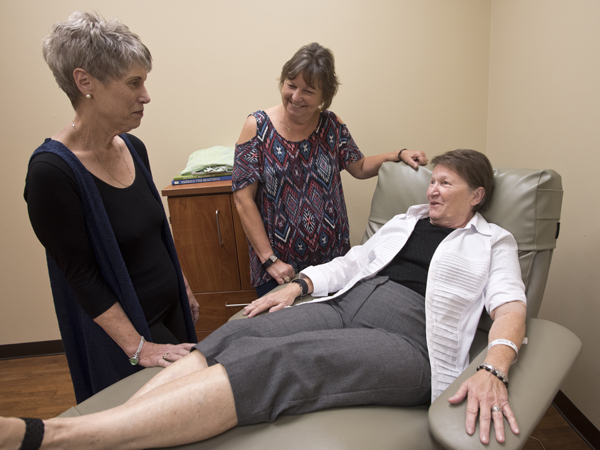
Hankins, seated, visits with friend Brenda Scafidi, left, and sister Beverly Sinele before her PET scan at the Jackson Medical Mall.
After the counting test and a series of other exams, Hankins' doctor diagnosed her with mild cognitive impairment, a condition that affects 15 percent of senior adults and can be an early sign of Alzheimer's disease. As part of her further evaluation, Hankins decided to enroll in Imaging Dementia - Evidence for Amyloid Scanning, or the IDEAS Study, a nationwide clinical trial offered by the MIND Center at the University of Mississippi Medical Center.
The MIND, or Memory Impairment and Neurodegenerative Dementia Center, combines research, brain imaging and genetic technologies to make discoveries about Alzheimer's disease. The MIND Center Clinic offers diagnosis and outpatient treatment for patients with memory loss and cognitive impairment, including dementia and Alzheimer's disease.

Huang
“Alzheimer's disease is a very devastating illness with no cure,” said Dr. Juebin Huang, assistant professor of neurology, MIND Center faculty and principal investigator for the UMMC IDEAS site. However, he says early detection and therapy can increase quality of life for patients.
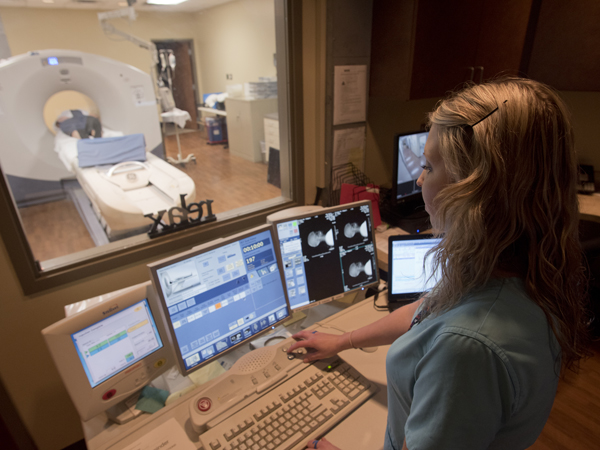
One goal of the IDEAS Study is to determine if using a PET (positron emission tomography) scan as part of the diagnostic process influences a physician's treatment recommendations and improves patient outcomes.
“Before a PET scan, clinicians inject a radioactive tracer that binds to a substance called amyloid plaques in the patient's brain. Using this imaging technique, we can see the tracers, which show us where the plaques are located,” Huang said.
Patients with Alzheimer's disease have ß-amyloid plaques and tangles - clumps of misfolded proteins - in the brain. When proteins have the wrong shape, they may not work properly and stick to other misfolded proteins. This botched molecular origami can pile up and disrupt the body's normal function. Scientists think these plaques and tangles damage neurons, leading to Alzheimer's symptoms.
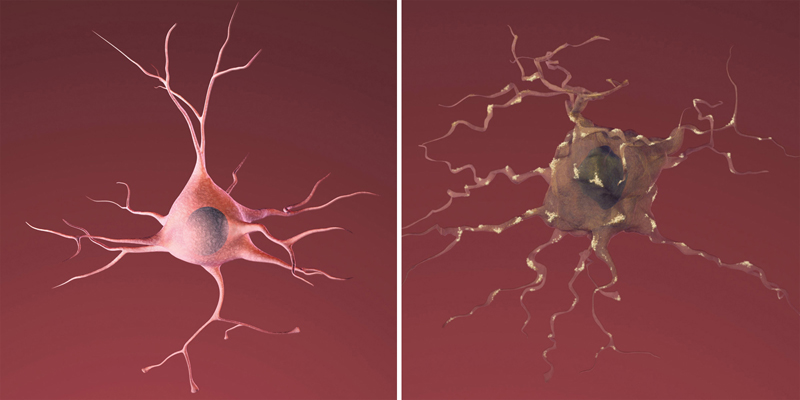
On the left is a healthy neuron. On the right, a neuron damaged by amyloid plaque, a sign of Alzheimer's Disease. (Image credit: National Institute on Aging)
Alzheimer's disease is the reason for memory loss in 50 to 70 percent of older patients, but stroke, head trauma, medication side effects and overuse of alcohol can also cause cognitive decline. Because there is no definitive test for Alzheimer's disease, Huang said it is a “diagnosis of exclusion,” meaning doctors rule out other possible causes for the symptoms first.
“There are studies that show a 20 to 30 percent misdiagnosis rate of Alzheimer's disease,” Huang said. With a false diagnosis, patients could get an inappropriate treatment that doesn't help or may even harm them. Using PET scans could eliminate some of the uncertainty.
“With an objective marker such as the amyloid PET imaging, physicians can make a more certain diagnosis and improve patient care and outcomes,” Huang said.
“We now have the technology, using a PET scan, to visualize amyloid protein in the brain of living patients which was previously only observable at autopsy,” said Dr. Tom Mosley, director of the MIND Center.
The Centers for Medicare and Medicaid Services currently covers PET scans for Alzheimer's evaluation only if the procedure is part of a research study, clinical trial or registry. The procedure is expensive, which limits accessibility. Hence, the other IDEAS goal is to learn if the scans improve patient outcomes enough to warrant their coverage by CMS.

Mosley
“My belief is that we will find that amyloid imaging provides a major improvement in both diagnosis and patient management and outcomes, and should be covered by insurance,” Mosley said.
“We hope the data from IDEAS will present CMS with good reason to reimburse diagnostic PET scans for Alzheimer's and dementia,” Huang said. “That coverage would give people better access to the scans.”
Hankins received her PET scan last week, just after returning from her Great Smoky Mountains trip with a group of friends. She said one of the highlights was going to Cades Cove, her “most favorite place on the planet.” Hankins plans to keep doing the things she loves as long as she can with the people that matter most: her family and friends.
“I have a great love for people,” Hankins said when discussing her participation in the IDEAS study. “I decided that this is something that I want to be involved in.
“I hope that over a period of time my family members and their grandchildren and great-grandchildren will benefit,” from the new knowledge generated by this study, Hankins said.
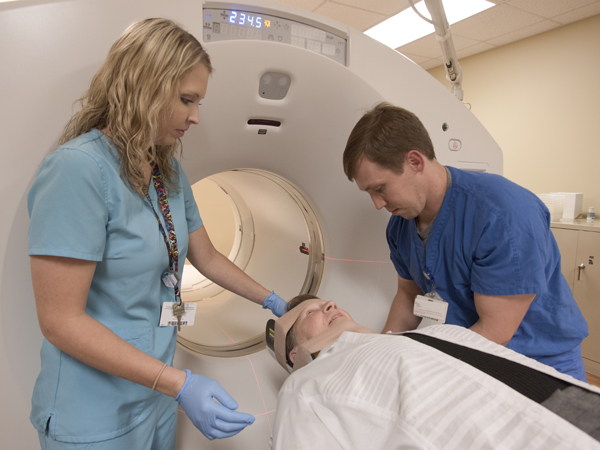
Registered nuclear medicine technologist Mary Beth Croisdale begins the PET scan of Hankins.
IDEAS is the first project in the MIND Center's Clinical Trials Program. Started in March 2016, the program will bring more opportunities to participate in industry-sponsored research studies related to Alzheimer's disease and dementia in addition to the MIND Center's population-based studies funded by the National Institutes of Health.
“The clinical trials program is critically important to the MIND Center,” Mosley said. “Clinical trials allow us to advance science in our search for effective treatments for Alzheimer's and related disorders and allows patients to have state-of-the-art care and access to the latest treatments before they are available to the general public.”

The IDEAS Study aims to enroll 18,000 participants who are at least 65 years old and have a diagnosis of cognitive impairment or dementia, but not Alzheimer's disease. The study is sponsored by the Alzheimer's Association, the American College of Radiology and the Centers for Medicare and Medicaid Services.


